Hydrogen is a common element found in all fossil fuels and all organic matter. Hydrogen is the simplest element, abundant, non-toxic and ultimate clean fuel. In its pure molecular form, H2, hydrogen is a colorless, odorless, nontoxic gas. Like oil and natural gas, hydrogen can be transported via pipeline or shipped in containers. It is the most efficient fuel and produces no emissions when used in a fuel cell. It can be produced from renewable resources and is not a greenhouse gas. When burned or used to power a fuel cell, hydrogen produces zero emissions besides water vapor. (For a more detailed description by NAAP)
Despite its simplicity and abundance, hydrogen doesn't occur naturally as a gas on the earth - it's always combined with other elements. Water, for example, is a combination of hydrogen and oxygen (H2O). It's been noted in numerous studies that hydrogen may be the only alternative fuel that can simultaneously reduce a country's dependence on foreign oil and significantly reduce greenhouse gases.
Hydrogen is the lightest gas and also the most energy-dense fuel per mass. One pound of hydrogen holds 52,000 Btu, three times the energy of a pound of gasoline. While hydrogen gas does not occur naturally on earth, it is easily produced in a variety of ways (link to “Hydrogen Production”).

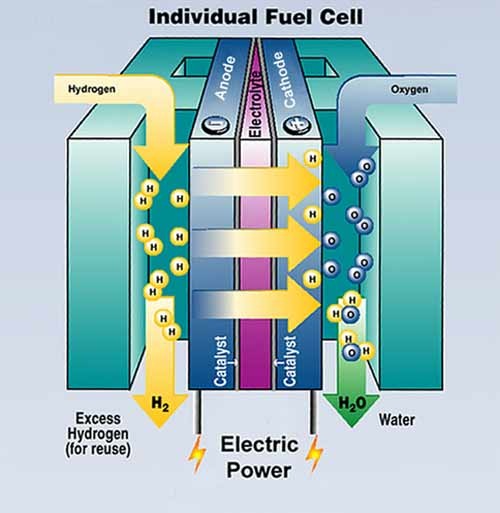
A fuel cell combines hydrogen and oxygen to produce electricity, heat, and water. Fuel cells are often compared to batteries. Both convert the energy produced by a chemical reaction into usable electric power. However, the fuel cell will produce electricity as long as fuel (hydrogen) is supplied, never losing its charge.
Hydrogen can be used in an internal combustion engine or a fuel cell to generate power. Significant advances have been made in the use of hydrogen as a transportation fuel and a fuel for power generation.
Efficiency of Hydrogen.
Hydrogen fuel cells with molten carbonate or solid oxide for their electrolyte membrane can use both the heat and electricity produced for extra efficiency, getting as high as 85 percent. Meanwhile, portable fuel cells like the polymer electrolyte membranes (PEM) used in fuel cell cars get anywhere from 50 percent to 60 percent efficiency, according to the U.S. Department of Energy. Most gasoline engines lose around 62 percent of their fuel energy just to wasted heat. hydrogen fuel is about three times more efficient than gasoline
The Problem is in the Production
Hydrogen may be the most plentiful element in the universe, but it needs to be extracted, a process that's expensive, time-consuming and takes an enormous amount of energy, but energy that could be created by solar, geothermal, hydro or other non-polluting means.
There are several potentially easier and promising ways to collect it, like harnessing hydrogen-producing algae and using methane from landfills.
A few more problems to solve:
-Hydrogen that escapes during the production process could erode the ozone layer even further and exacerbate global warming.
-Storage and distribution: Hydrogen needs to be stored and transported under high pressure. Hydrogen is highly flammable, but unlike gas, it has no smell. Sensors must be used to detect a leak before hydrogen can combust. Another issue is refueling stations. Hydrogen fuel producers must be willing to put a hydrogen station on virtually every corner.
http://www.nrel.gov/learning/eds_hydro_production.html
http://www.nrel.gov/hydrogen/proj_production_delivery.html
http://www.undeerc.org/NCHT/midH2works/default.aspx
http://chemeng-processing.blogspot.com/2010/05/hydrogen-production-by-steam-reforming.html
http://www.renewableenergyworld.com/rea/tech/hydrogen
http://www.airproducts.com/industries/energy/hydrogen-energy.aspx
http://www.fchea.org/index.php?id=46
http://astro.unl.edu/naap/hydrogen/levels.html
http://dsc.discovery.com/energy/energy-power/hydrogen-fuel-cell-energy-ratio.html